Virtual screening
hAR
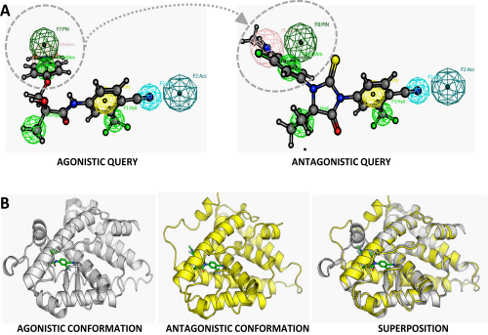
Unravelling the mechanisms involved in castration- and therapy-resistant prostate cancer has led to a renewed interest in androgen receptor (AR) targeted therapeutics. Antiandrogens that block the activity of the AR thus remain a valid therapeutic option. However they have to be more effective or display a distinct mechanism of action or binding mode compared to the compounds, bicalutamide and hydroxyflutamide which are in current clinical use. For that reason, the second-generation antiandrogen, MDV3100, was developed. MDV3100, however, shares its 4-cyano-3-(trifluoromethyl)phenyl group with bicalutamide and hydroxyflutamide required for binding to the AR. In collaboration with Prof. Frank Claessens, Laboratory for Molecular Endocrinology, Faculty of Medicine, K.U.Leuven, Belgium, we used a combined strategy to find new antagonist structures distinct from the 4-cyano-3-(trifluoromethyl)phenyl group to avoid cross-resistance for these compounds and to find structures without agonist activity on mutant ARs (AR W741C and AR T877A). We found two novel chemotypes with AR-antagonistic activity (IC50 of 3-6 µM) by virtual screening and confirmed their biological activity in an androgen-responsive reporter assay. The design of our computational approach was validated by the observation of strongly reduced or lack of agonistic activity on two mutant ARs, AR W741C and AR T877A. Further structural derivatization to optimize the potency of these compounds can render these chemotypes into very promising, alternative AR-antagonists for prostate cancer therapy. This work has been published in ChemMedChem
(publication).