Prediction and design
Nanocrystal
Biomineralization of a Cadmium Chloride Nanocrystal by a Designed Symmetrical Protein
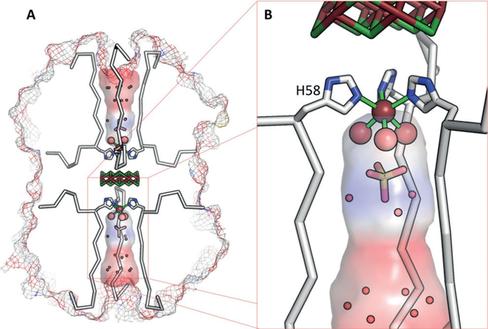
We have engineered a metal binding site into the novel artificial beta-propeller protein Pizza. This new Pizza variant carries two nearly identical domains per polypeptide chain, and forms a trimer with three-fold symmetry. The designed single metal ion binding site lies on the symmetry axis, bonding the trimer together. Two copies of the trimer associate in the presence of cadmium chloride in solution, and very high resolution X-ray crystallographic analysis reveals a nano-crystal of cadmium chloride, sandwiched between two trimers of the protein. This nano-crystal, containing seven cadmium ions lying in a plane and twelve interspersed chloride ions, is the smallest reported to date. Our results indicate the feasibility of using rationally-designed symmetrical proteins to biomineralize nano-crystals with useful properties. This work has been published in Angew. Chem. Int. Ed. (publication).This work has been highlighted by RIKEN Press Release issued on July 2, 2015. Engineering the world’s smallest nanocrystal
The overall structure of the designed protein, nvPizza2-S16H58, is shown below on the left panel. A close-up view of the cadmium chloride nanocrystal is shown on the right panel. All figures reprinted from Voet et al., DOI:10.1002/anie.201503575
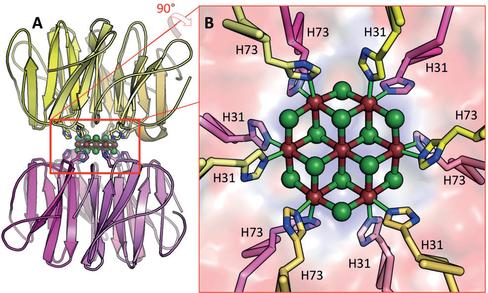
A cross-section through the nvPizza2-S16H58 structure is shown below on the left panel. A close-up view of the designed binding site with cadmium ion coordinated by His58 and water is shown on the right panel.