Virtual screening
Sumoylation
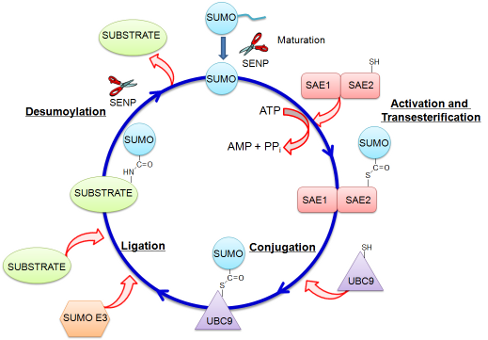
Sumoylation is a post-translational modification of proteins that regulates important cellular functions, such as cell proliferation, chromosome packing and dynamics, DNA replication and repair, genome integrity, nuclear transport and signal transduction. Sumoylation of target proteins consists of the covalent attachment of one of the Small Ubiquitin-like MOdifier (SUMO) proteins to a lysine located in a consensus sequence of the target proteins by means of an enzymatic cascade involving several enzymes: the E1 activating enzyme (AOS1/UBA2), the E2 conjugating enzyme (UBC9) and sometimes an E3 ligase. Emerging evidence suggests that sumoylation plays a general role in regulating protein-protein interactions (PPIs). This is mediated via the recognition of SUMO proteins by a SUMO interaction motif (SIM). Given its role in many important cellular processes, the sumoylation pathway has also been linked to a significant number of pathogenicities including neurodegenerative diseases and cancer. This makes sumoylation a novel drug target. We have been working on the discovery of small molecule inhibitors of these proteins involved in the sumoylation pathway, SENP, SUMO E1, Ubc9 as well as small molecule protein-protein interaction inhibitors (SMIPPIs), such as SUMO:SIM.
 Please refer to the following items for details:
Please refer to the following items for details:
 Please refer to the following items for details:
Please refer to the following items for details: