研究概要
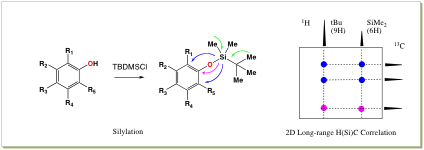
- We have developed novel 2D long-range range H(X)C and H(C)X triple-resonance NMR experiments at natural abundance. Available X is one-half spin nucleus such as 15N, 29Si, 31P, 77Se, 119Sn, 195Pt. In structural elucidation, assignment of hydroxyl group position is an important step. We have developed a new method for determining the position of phenolic hydroxyl groups through silylation and application of 2D long-range H(Si)C triple-resonance NMR experiment. We chose tert-butyldimethylsilyl (TBDMS) group as a silyl group because of high sensitivity in 1H by detection of two singlet methyl signals with six and nine equivalent protons, and easiness of handling as protective group.
-
H(Si)C experiment
- Malon, M., Takahashi, S., and Koshino, H., A new method for determining positions of phenolic hydroxyl groups through silylation and application of H(Si)C triple-resonance NMR experiments, Tetrahedron Lett., 48, 7586-7590 (2007)
-
H(P)C and H(C)P experiments
- Malon, M. and Koshino, H., H(C)P and (H(P)C triple-resonance experiments at natural abundance employing long-range couplings, Magn. Reson. Chem., 45, 770-776 (2007)
-
H(Se)C and H(C)Se experiments
- Malon, M., Imakubo, T., and Koshino, H., 1H, 13C and 77Se NMR study of tetraselenafulvalene derivatives supported by novel H(Se)C and H(C)Se triple-resonance experiments at natural abundance, Magn. Reson. Chem., 46, 150-155 (2008)
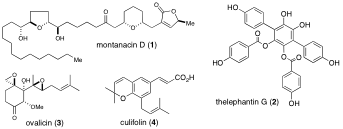
- The aims of our research project are development of new chemical and synthetic methods for molecular characterization. As a main theme, we have continued synthesis of a biologically active natural product whose structure is difficult to determine by spectroscopic methods especially. The structure of natural products such as an antitumor annonaceous acetogenin 1 and a p-terphenyl 2 with an inhibition potential of TNF-α production was clarified by systematic total synthesis. Furthermore, syntheses of an angiogenesis inhibitor 3 and the bioactive principles in Brazilian propolis (e. g. 4) were also performed, and their biological activities were evaluated.
-
acetogenins
- Takahashi, S., Hongo, Y., Tsukagoshi, Y., and Koshino, H., Structural determination of montanacin D by total synthesis, Org. Lett., 10, 4223-4226 (2008)
- Takahashi, S., Takahashi, R., Hongo, Y., Koshino, H., Yamaguchi, K., and Miyagi, T., Synthesis of all possible isomers corresponding to the proposed structure of montanacin E, and their antitumor activity, J. Org. Chem., 74, 6382-6385 (2009)
-
p-terphenyls
- Ye, Y. Q., Koshino, H., Onose, J., Yoshikawa, K., Abe, N., and Takahashi, S., First total synthesis of vialinin A, a novel and extremely potent inhibitor of TNF-α production, Org. Lett., 9, 4131-4134 (2007)
- Ye, Y. Q., Koshino, H., Onose, J., Negishi, C., Yoshikawa, K., Abe, N., and Takahashi, S., Structural revision of thelephantin G by total synthesis and inhibitory activity against TNF-α production, J. Org. Chem., 74, 4642-4645 (2009)
-
ovalicin
- Takahashi, S., Hishinuma, N., Koshino, H., and Nakata, T., Synthesis of ovalicin starting from D-mannose, J. Org. Chem., 70, 10162-10165 (2005)
-
propolis
- Tani, H., Hasumi, K., Tatefujji, T., Hashimoto, K., Koshino, H., and Takahashi, S., Inhibitory activity of Brazilian green propolis components and their derivatives on the release of cys-leukotrienes, Bioorg. Med. Chem., 18, 151-157 (2010)
-
acetogenins
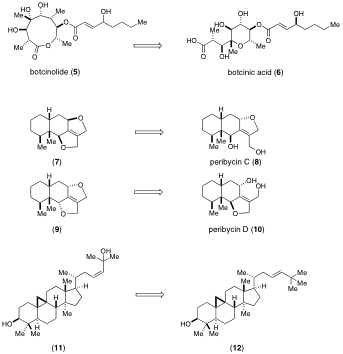
- We are interested in development of precise structural determination methods and reliable application of them to natural products and related synthetic compounds. It is well known that the NMR chemical shift is useful information and sensitive to the stereochemistry of organic molecules. We developed CAST/CNMR for highly accurate 13C NMR chemical shift prediction with effective consideration of stereochemistry and ring structures in collaboration with NII (National Institute of Informatics). For example, we reported the structural revision of botcinolide 5, eremophilane sesquiterpenoid peribysins C 8 and D 10, and many terpenoids with (3Z)-2-methyl-3-penten-2-ol moiety such as (23Z)-cycloart-23-ene- 3β,25-diol. In case of (23Z)-cycloart-23-ene-3β,25-diol 11 and 23E isomer 12 we synthesized authentic samples and studied NMR chemical shifts in several solvent systems.
-
botcinolides
- Tani, H., Koshino, H., Sakuno, E., and Nakajima, H., Botcinins A, B, C, and D, metabolites produced by Botrytis cinerea, and their antifungal activity against Magnaporthe grisea, a pathogen of rice blast disease, J. Nat. Prod., 68, 1768-1772 (2005)
- Tani, H., Koshino, H., Sakuno, E., Cutler, H. G., and Nakajima, H., Botcinins E and F and botcinolide from Botrytis cinerea and structural revision of botcinolides, J. Nat. Prod., 69, 722-725 (2006)
-
CAST/CNMR and application
- Satoh, H., Koshino, H., Uzawa, J., and Nakata, T., CAST/CNMR: highly accurate 13C NMR chemical shift prediction system considering stereochemistry, Tetrahedron, 59, 4539-4547 (2003)
- Satoh, H., Koshino, H., Uno, T., Koichi, S., Iwata, S., and Nakata, T., Effective consideration of ring structures in CAST/CNMR for highly accurate 13C NMR chemical shift prediction, Tetrahedron, 61, 7431-7437 (2005)
- Koshino, H., Satoh, H., Yamada, T., and Esumi, Y., Structural revision of peribysins C and D, Tetrahedron Lett., 47, 4623-4626 (2006)
- Takahashi, S., Satoh, H., Hongo, Y., and Koshino, H., Structural revision of terpenoids with a (3Z)-2-methyl-3-penten-2-ol moiety by synthesis of (23E)-and (23Z)-cycloart-23-ene-3β, 25-diols, J. Org. Chem., 72, 4578-4581 (2007)
- We provide a wide range of mass spectrometry-related technological and scientific support to the scientists including chemists and biologists work with any kind of organic molecules (small and large, except for proteomic samples). We maintain a choice of instrumentation to meet diverse research needs for characterization of organic molecules. Our supporting activity includes training of open-access facility user, accurate mass analysis of a variety of organic compounds, molecular weight and elemental composition analysis, structural characterization of unknown compounds, characterization of mixtures, support on spectral and data interpretation, consulting and comprehensive support on experimental design and execution for solving any kind of qualitative and quantitative problems that can be solved by mass spectrometry. Taking the advantage of our expertise in mass spectrometry, we also engage in scientific collaboration with scientists (inside/outside RIKEN).
- Our research activity on mass spectrometry includes exploration of new methods and concepts in mass spectrometry and their applications. The goal of those basic researches is to provide better mass spectrometry tools to the scientific community in future.
- Examples
- Identification of small molecules (application/collaboration)
- Characterization of isomeric mixtures (application/collaboration)
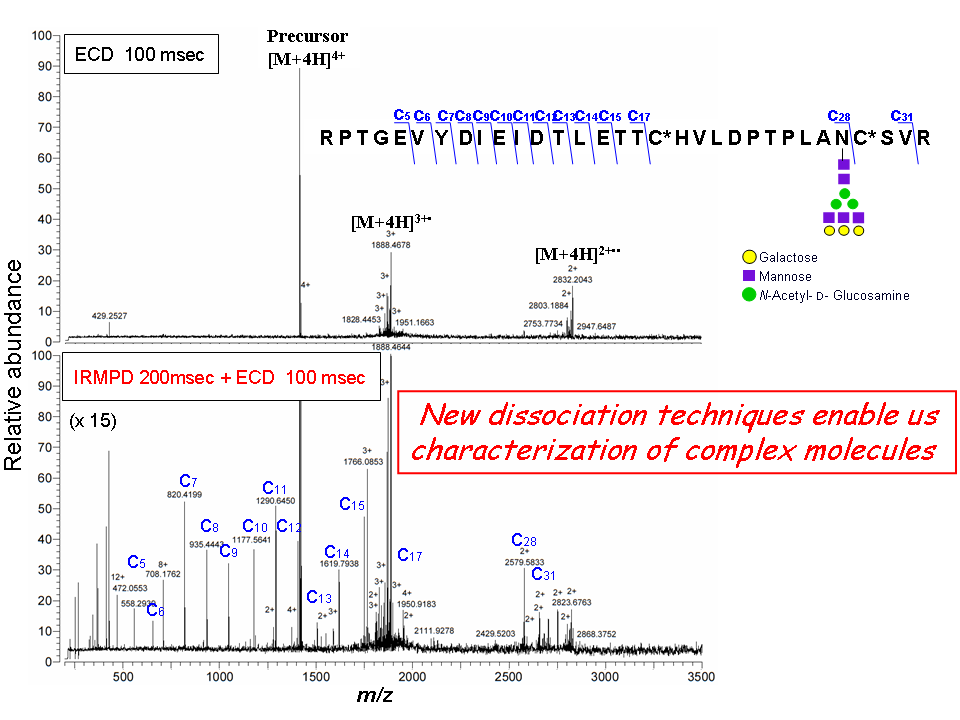
- Dissociation method for structural analysis (methodological/basic)
- Hongo, Y., Nakamura, T., and Sato, A., Electron capture dissociation of triantennary complex-type N-glycosylated peptides: a case of suppressed peptide backbone cleavage, J. Mass Spectrom. Soc. Jpn., 55, 77-82 (2007)
- Hongo, Y., Sato, A., and Nakamura, T., Factors governing peptide backbone cleavages in electron capture dissociation of triantennary complex-type N-glycosylated peptide, J. Mass Spectrom. Soc. Jpn., 55, 279-285 (2007)