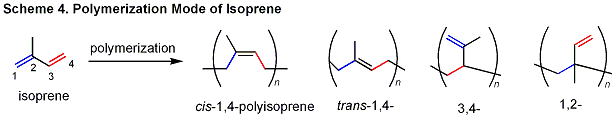| 1. New Catalysts for Regio- and Stereospecific Polymerization |
|
Compared to most transition metals which can show variable
(different) oxidation states, rare earth metals usually adopt the +3 oxidation
state as the most stable oxidation state, which is not easily changed to
other oxidation states under normal conditions. This nature makes rare
earth metals unique candidates for the formation of true "single-site"
polymerization catalysts. Aiming towards the creation of new, high performance
polymer materials, a part of our research program focuses on developing
highly active and selective polymerization catalysts on the basis of the
unique character of the rare earth metal complexes.
|
|
| 1-1. Development of Effective Catalyst for Syndiospecific Styrene-Ethylene Copolymerization |
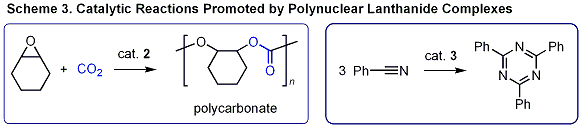
Syndiotactic polystyrene (sPS, see Scheme 1), is a very promising polymer because of its excellent
resistance to heat and chemicals. A drawback that limits the application
of sPS, however, is its brittleness. Since the discovery of sPS, extensive
studies on the copolymerization of styrene with ethylene have been carried
out to improve the toughness of this material. However, such attempts to
obtain a styrene-ethylene copolymer having syndiotactic styrene-styrene
sequences were not successful.
In striking contrast, our original catalyst system prepared
from half-sandwich scandium complex 1 and [Ph3CB][(C6F5)4] exhibits excellent activity and selectivity for syndiospecific styrene-ethylene
copolymerization. When the copolymerizations were carried out in the presence
of ethylene and styrene by using this catalyst system, multi-block styrene-ethylene
copolymers consisting of sPS sequences connected by polyethylene units
were obtained. This is the first example of syndiospecific copolymerization of styrene with ethylene. Also A-B diblock styrene-ethylene copolymers with a sPS block could be
synthesized by a sequential polymerization of styrene and ethylene in the
presence of the same catalyst system. |
 |
Reference
(1) J. Am. Chem. Soc. 2004, 126, 13190. <ACS e-reprint> |
|
| 1-2. Development of a New Catalyst for Norbornene-Ethylene Copolymerization |
| Cyclic olefin copolymers (COCs) have recently attracted significant
interest as one of the most important engineering plastics because of their
many desirable properties such as thermal stability, good transparency,
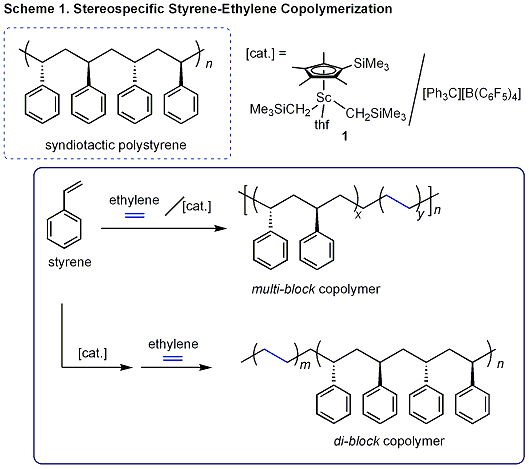
and chemical resistance. We recently found that cationic scandium species
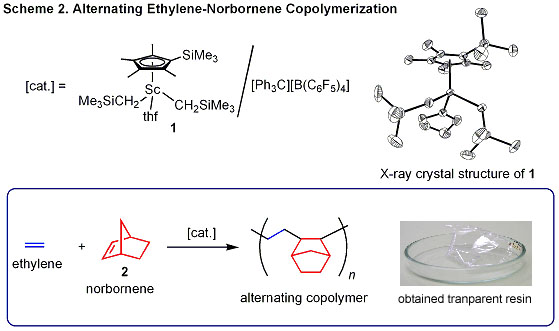
generated from half-sandwich scandium complex 1 and [Ph3CB][(C6F5)4] can act as an excellent catalyst system for alternating copolymerization of norbornene and ethylene, providing very transparent plastics (Scheme 2). This catalyst system
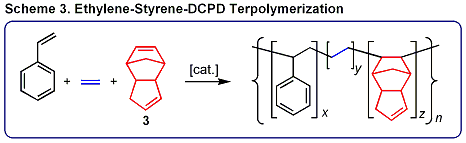
is also effective for ethylene-dicyclopentadiene (DCPD, 3)-styrene terpolymerization (Scheme 3), constituting the first example of terpolymerization of ethylene,
styrene, and a cyclic olefin. |
 |
References
(1) Angew. Chem. Int. Ed. 2005, 44, 962. (2) Maclomolecules 2005, 38, 6767. <ACS e-reprint> |
|
| 1-3. Development of High-Performance Catalysts for Isoprene Polymerization |
| Isoprene has a conjugated diene moiety, and its polymerization
can provide various isomeric polymers (cis-1,4-; trans-1,4-; iso-, syndio-, or atactic 3,4-), depending upon how the C-C double bonds react.
Therefore, it is critical to control the regio- and stereospecificities,
in addition to molecular weight in order to obtain a high-quality polyisoprene
having desirable properties. |
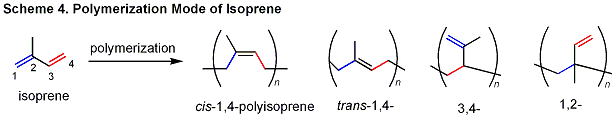 |
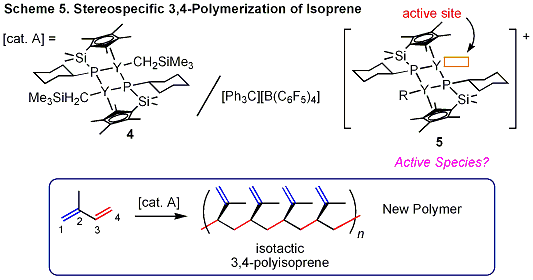
We recently found that a catalyst system prepared from the
binuclear half-sandwich yttrium complex 4, in which the two metal centers are bridged by two phosphido ligands, exhibits excellent activity and unprecedented isospecific 3,4-selectivity for isoprene polymerization, which affords for the first time a polyisoprene polymer with almost perfect isotactic 3,4-microstructure, high molecular weight, and unimodal narrow molecular weight distribution (Scheme 5). The obtained isotactic 3,4-polyisoprene is a new polymer and its applications will be explored. |
 |
Cis-1,4-polyisoprene is the main component of natural rubber
(NR). One of the drawbacks of natural rubber, however, is that it contains
allergic proteins and its molecular weight distribution is usually rather
broad. On the other hand, the Cis-1,4-microstructure content of conventional
synthetic polyisoprenes (isoprene rubber) is generally lower (up to 98%)
than that of NR, and their physical properties are not as good as those
of NR, despite just a 2% of difference.
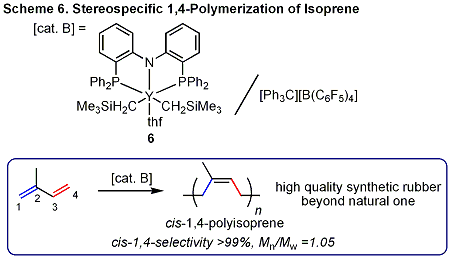
We recently succeeded to synthesize a novel, non-metallocene yttrium complex 6 (Scheme 6) that exhibits excellent "livingness" and an extremely
high cis-1,4 regio- and stereoselectivity for the polymerization of isoprene.
This new catalytic system can provide an almost perfectly cis-1,4-regulated polyisoprene (nearly 100%) with a very narrow
molecular weight distribution (<1.1) even at room or higher temperatures. The newly synthesized cis-1,4-polyisoprene
is a very promising new polymer material suitable for a large number of
applications, such as high-quality tires. |
 |
References
(1) J. Am. Chem. Soc. 2005, 127, 14562. <ACS e-reprint> (2) Tetrahedron 2003, 59, 10525. |
|
| >>Return |
|