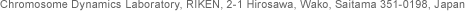(1) Molecular mechanisms of action of SMC protein complexes
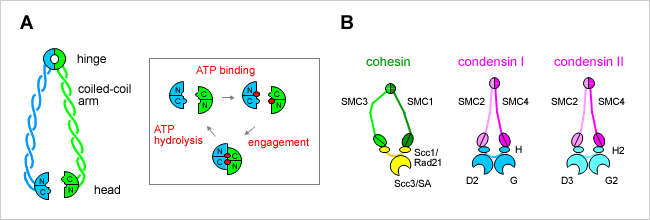
SMC proteins represent a large family of chromosomal ATPases conserved from bacteria to humans. SMC proteins form a V-shaped dimer with two long coiled-coil arms, each having an ATP-binding ‘head’ domain at its distal end (Figure A). Most bacteria and archaea have a single SMC protein that forms a homodimer. Individual eukaryotic organisms have at least six SMC family members that form three heterodimers in specific combinations. Each dimer further associates with a distinct set of non-SMC regulatory subunits to form a functional holocomplex. For example, a pair of SMC1 and SMC3 constitutes the core of the cohesin complexes, whereas SMC2 and SMC4 function as central components of the condensin complexes (Figure B).
We are interested in understanding how SMC proteins and SMC protein complexes might work at a mechanistic level. It is thought that the cycle of ATP binding and hydrolysis modulates conformational changes of the V-shaped molecule and regulates its interaction with non-SMC subunits. The detailed mechanisms of action of SMC proteins remain to be determined, however. We use condensin and cohesin complexes (either purified from native sources or reconstituted from recombinant subunits), and compare and contrast their molecular actions in vitro. A bacterial SMC protein is also used as a model system for studying the basics of SMC actions. In addition to conventional biochemical approaches, we take structural and biophysical approaches.