Analysis of functions of molecular chaperones and its
applications
|
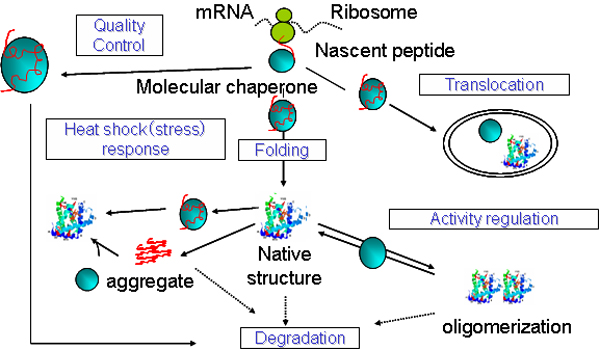
Molecular chaperones are ubiquitous
proteins that assist folding, assembly, transport and degradation of proteins
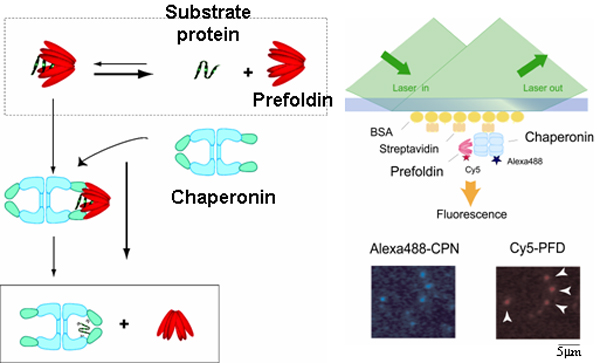
within the cell. Our group are interested in the molecular mechanism of prefoldin, one of molecular chaperone, which assists a
protein folding by capturing folding intermediate and transferring it to chaperonin. We are also interested in the application of
molecular chaperone in Biotechnology field. Various research using these
multidisciplinary approaches are carried out mainly with biochemical and
biophysical experimental techniques including single molecule imaging.
|
References
|
(1) |
Tamotsu Zako, Miya
Yoshimoto, Hiroshi Hyodo, Hidehiro
Kishimoto, Masaaki Ito, Kazuhiro Kaneko, Kohei Soga and Mizuo Maeda
"Cancer-targeted Near Infrared imaging using rare earth ion-doped
ceramic nanoparticles" Biomater.
Sci. 3, 59-64 (2015) |
|
(2) |
Tamotsu Zako and Mizuo Maeda "Application of biomaterials for the detection of amyloid aggregates" Biomater. Sci. 2, 951-955 (2014) |
|
(3) |
Eisuke Takai, Ken Uda,
Shuhei Matsushita, Yui Shikiya, Yoichi Yamada, Tamotsu Zako,
Mizuo Maeda and Kentaro Shiraki "Cysteine Inhibits
Amyloid Fibrillation of the Lysozyme
and Directs the Formation of Small Worm-like Aggregates through Non-Covalent
Interactions" Biotech. Prog. 30, 470-478
(2014) |
|
(4) |
Eisuke Takai,
Gai Ohashi, Tomonori
Yoshida, Karin M. Sorgjerd, Tamotsu Zako, Mizuo Maeda, Katsuhisa Kitano and Kentaro Shiraki "Degeneration of Amyloid-b
Fibrils by Low Temperature Atmospheric Pressure Plasma in Aqueous
Solution" Appl. Phys. Lett. 104, 023701 (2014) |
|
(5) |
Eisuke Takai,
Ken Uda, Tomonori Yoshida, Tamotsu Zako, Mizuo Maeda, Yoichi
Yamada and Kentaro Shiraki
"Cysteine Inhibit Fibrillization
and Cytotoxicity of Amyloid-beta
40 and 42: Implication for Contribution of Thiophilic
Interaction” Phys. Chem. Chem. Phys. 16, 3566-3572 (2014) |
|
(6) |
Aline C.C. Rotzetter,
Christoph M. Schumacher, Tamotsu Zako, Wendelin J. Stark and Mizuo Maeda "Rapid Surface- Biostructure
Interaction Analysis using strong Metal-based Nanomagnets"
Langmuir 29, 14117-14123 (2013) |
|
(7) |
Helene Vignaud,
Claude Bobo, Ioan Lascu, Karin M. Sorgjerd,
Tamotsu Zako, Mizuo
Maeda, Benedicte Salin,
Sophie Lecomte and Christophe Cullin
"A structure-toxicity study of Abeta42 reveals a new anti-parallel
aggregation pathway" PLoS ONE 8, e80262 (2013) |
|
(8) |
Tong Bu, Tamotsu Zako,
Masahiro Fujita and Mizuo Maeda "Detection of
DNA Induced Gold Nanoparticle Aggregation with Dark
Field Imaging" Chem. Commun. 49, 7531-7533
(2013) |
|
(9) |
Erika Tashiro,
Tamotsu Zako, Hideki Muto, Yoshinori Itoo, Karin Sorgjerd, Naofumi Terada, Akira Abe, Makoto Miyazawa, Akira
Kitamura, Hirotake Kitaura,
Hiroshi Kubota, Mizuo Maeda, Takashi Momoi, Sanae M.M. Iguchi-Ariga, Masataka Kinjo, and Hiroyoshi Ariga "Prefoldin protects neuronal cells from polyglutamine toxicity by preventing aggregation
formation" J. Biol. Chem.
288, 19958-19972 (2013) |
|
(10) |
Karin M Sorgjerd,
Tamotsu Zako, Masafumi Sakono, Peter C Stirling,
Michel R Leroux, Takashi Saito, Per Nilsson, Misaki Sekimoto, Takaomi C Saido, and Mizuo Maeda "
Human Prefoldin Inhibits Amyloid-β
(Aβ) Fibrillation and Contributes to Formation of
Nontoxic Aβ Aggregates" Biochemistry, 52,
3532-3542 (2013) |
|
(11) |
Naofumi Terada, Tamotsu Zako and Mizuo Maeda "Photon
counting histogram using numerical data of point spread function" Jpn. J. Appl. Phys. 52, 038001 (2013) |
|
(12) |
Masafumi Sakono,
Arata Utsumi, Tamotsu Zako, Tetsuya Abe, Masafumi Yohda and Mizuo Maeda,
"Formation of non-toxic Aβ fibrils by small heat
shock protein under heat-stress conditions" Biochem
Biophys Res Commun. 430,
1259-64 (2013) |
|
(13) |
Ryoko Watanabe-Tamaki, Atsushi
Ishikawa, Takuo Tanaka, Tamotsu Zako
and Mizuo Maeda, "DNA-Templating
Mass Production of Gold Trimer
Rings for Optical Metamaterials" J. Phys.
Chem. C, 116, 15028-15033 (2012) |
|
(14) |
Tamotsu Zako, Masafumi
Sakono, Takahiro Kobayashi, Karin Sorgjerd, K. Peter R.
Nilsson, Per Hammarstrom, Mikael
Lindgren and Mizuo Maeda "Cell interaction
study of amyloid using luminescent conjugated polythiophene: Implication that amyloid
cytotoxicity is correlated with prolonged cellular
binding" ChemBioChem 13, 358-363 (2012) |
|
(15) |
Katarzyna Maria Psonka-antonczyk, Julien Duboisset, Bjorn Torger Stokke, Tamotsu Zako, Takahiro Kobayashi, Mizuo Maeda, Sofie Nystrom, Jeff Mason, Per Hammarstrom,
K. Peter. R. Nilsson, Mikael Lindgren "Nanoscopic and photonic ultrastructural
characterization of two distinct insulin amyloid
states" Int. J. Mol. Sci. 13, 1461-1480 (2012) |
|
(16) |
Masafumi Sakono, Tamotsu Zako,
Masafumi Yohda and Mizuo Maeda "Amyloid
oligomer detection by immobilized molecular
chaperone" Biochem. Eng. J. 61, 28-33 (2012) |
|
(17) |
Masafumi Sakono, Tamotsu Zako
and Mizuo Maeda
"Naked-eye Detection of Amyloid Aggregates
using Gold Nanoparticles Modified with Amyloid Beta Antibody" Anal. Sci. 28, 73-76 (2012) |
|
(18) |
Masafumi Sakono,
Shigenori Akiyama, Tamotsu Zako, Shujiro Sakaki, Tomonori Waku,
Naoki Tanaka and Mizuo Maeda "Immobilized
Insulin Amyloid Enhances Cell Adhesion and
Proliferation due to Interaction with Fibronectin"
Chem. Lett., 40, 315-317 (2011) |
|
(19) |
Masafumi Sakono,
Shigenori Akiyama, Tamotsu Zako, Shujiro Sakaki, Tomonori Waku,
Naoki Tanaka and Mizuo Maeda "Application of
two morphologically different fibrillar and
filamentous insulin amyloids as a biomaterial for
cell culture surfaces" React. Funct. Polym. 71, 324-328 (2011) |
|
(20) |
Masafumi Sakono,
Tamotsu Zako, Srdja Drakulic, Jose M Valpuesta, Masafumi Yohda and Mizuo Maeda "Size-selective recognition of gold nanoparticles by a molecular chaperone" Chem. Phys. Lett. 501, 108-112 (2010) |
|
(21) |
Takahito Ohshiro,
Tamotsu Zako, Ryoko Wanatabe-Tamaki, Takuo Tanaka
and Mizuo Maeda "A Facile Method Towards
Cyclic Assembly of Gold Nanoparticles Using DNA
Template Alone" Chem. Comm. 46, 6132-6134 (2010) |
|
(22) |
Muhamad Sahlan,
Taro Kanzaki,Tamotsu Zako,
Mizuo Maeda and Masafumi Yohda "Analysis of the interaction mode between hyperthermophilic archaeal
group II chaperonin and prefoldin
using a platform of chaperonin oligomers
of various subunit arrangements" Biochimica et
Biophysica Acta -
Proteins & Proteomics, 1804, 1810-1816 (2010) |
|
(23) |
Muhamad Sahlan,
Tamotsu Zako, Phan The
Tai, Akashi Ohtaki, Keiichi Noguchi, Mizuo Maeda, Hideyuki Miyatake,
Naoshi Dohmae and Masafumi Yohda
"Thermodynamic Characterization of the Interaction between Prefoldin and Group II Chaperonin"
J Mol. Biol. 399, 628-636 (2010) |
|
(24) |
Zako, T., Hyodo,
H., Tsuji, K., Tokuzen, K., Kishimoto,
H., Ito, M., Kaneko, K., Maeda, M. and Soga, K."Development of Near
Infrared-Fluorescent Nanophosphors and Applications
for Cancer Diagnosis and Therapy" J. Nanomaterials,
2010, 491471 (2010) |
|
(25) |
Bando, K., Zako, T., Sakono, M., Maeda, M., Wada, T., Nishijima,
M., Fukuhara, G., Yang, C., Mori, T., Pace, T.C.S.,
Bohne, C. and Inoue, Y. "Bio-Supramolecular Photochirogenesis
with Molecular Chaperone: Enantiodifferentiating Photocyclodimerization of 2-Anthracenecarboxylate
Mediated by Prefoldin" Photochem.
Photobiol. Sci., 9, 655 - 660 (2010) |
|
(26) |
Sakono, M. and Zako,
T. "Amyloid oligomers:
Formation and toxicity of Abeta oligomers"
FEBS J. 277, 1348-1358(2010) |
|
(27) |
Zako, T. "Amyloid
oligomers" FEBS J. 277, 1347 (2010) |
|
(28) |
Zako, T., Banba,
S., Sahlan, M., Sakono,
M., Terada, N., Yohda, M. and Maeda, M. "Hyperthermophilic archaeal prefoldin shows refolding activity at low
temperature" Biochem. Biophys.
Res. Commun. 391, 467-470 (2010) |
|
(29) |
Zako, T., Sakono,
M., Hashimoto, N., Ihara, M. and Maeda, M. "Bovine Insulin Filaments
Induced by Reducing Disulfide Bonds Show a Different Morphology, Secondary
Structure and Cell Toxicity from Intact Insulin Amyloid
Fibrils" Biophys. J., 96, 3331-3340 (2009) |
|
(30) |
Zako,
T., Nagata, H., Terada, N., Utsumi, A.,
Sakono, M., Yohda, M., Ueda, H., Soga, K. and Maeda, M. "Cyclic RGD Peptide-Labeled Upconversion Nanophosphors for
Tumor Cell-Targeted Imaging" Biochem. Biophys. Res. Commun., 381,
54-58 (2009) |
|
(31) |
Sakono, M., Zako, T., Ueda, H., Youda, M.
and Mizuo, M. “Formation of highly toxic soluble amyloid beta oligomers by the
molecular chaperone prefoldin” FEBS Journal, 275,
5982-5993 (2008) |
|
(32) |
Zako, T., Iizuka, R., Kanzaki, T., Maeda,
M. and Yohda, M. “Chaperonin
and prefoldin - Two molecular chaperones that work
cooperatively in archaea and eukaryote” in Heat shock proteins
:New Research (Edited by Emma Moreland and Camille Vincent); pp. 393-416 Nova
Science Publishers, Inc., |
|
(33) |
Suzuki
M, Ueno T, Iizuka R, Miura T, Zako
T, Akahori R, Miyake T, Shimamoto
N, Aoki M, Tanii T, Ohdomari
I, Funatsu T, “Effect of the C-terminal truncation
on the functional cycle of chaperonin groel: Implicatoin that the
C-terminal region facilitates the trasition from
the folding-arrested to the folding-competent state” J. Biol. Chem., 283, 23931-23939
(2008) |
|
(34) |
Zako, T.,
Nagata, H., Terada, N., Sakono, M, Soga, K and
Maeda, M. ”Improvement
of dispersion stability and characterization of upconversion
nanophosphors covalently modified with PEG as a
fluorescence bioimaging probe” J. Materials Sci.
43, 5325-5330, (2008) |
|
(35) |
Kurimoto
E, Nishi Y, Yamaguchi Y, Zako T, Iizuka R, Ide N, Yohda M, Kato K. ”Dynamics of group II chaperonin and prefoldin probed
by (13)C NMR spectroscopy.” Proteins, 70, 4, 1257-1263 (2008) |
|
(36) |
Miyake,
T, Tanii, T., Kato, K., Zako,
T., Funatsu, T. and |
|
(37) |
Horiuchi, H., Iwami, N., Tachibana, F., Ohtaki,
A., Iizuka, R., Zako, T.,
Oda, M., Yohda, M. and Tani, T. "Complex formation of CdSe/ZnS/TOPO nanocrystal vs.
molecular chaperone in aqueous solution by hydrophobic interaction" J.
Luminescence, 127, 192-197 (2007) |
|
(38) |
Zako T, Murase Y, Lizuka R, Yoshida T, Kanzaki T, Ide N, Maeda M, Funatsu T, Yohda M, “Localization
of Prefoldin Interaction Sites in the Hyperthermophilic Group II Chaperonin
and Correlations between Binding Rate and Protein Transfer Rate”, J. Mol.
Biol., 364, 110-120 (2006) |
|
(39) |
Yoshida
T, Kanzaki T, Iizuka R, Komada T, Zako T, Suzuki R,
Maruyama T, Yohda M, “Contribution
of The C-terminal region to the thermostability of
the group II chaperonin
from the hyperthermophilic archaeum,
Thermococcus sp. strain KS-1”, Extremophiles,
5, 451-459 (2006) |
|
(40) |
Terada
N, Tadakuma H, Ishihama
Y, Yamagihsi M, Zako T, Funatsu T, “Analysis
of Nuclear Microenvironments by Translational Diffusion of GFP Using Fluorescence
Correlation Spectroscopy”, Bioimages, 13, 1-10
(2005) |
|
(41) |
Iizuka R,
Yoshida T, Ishii N, Zako T, Takahashi K, Maki K, Inobe T, Kuwajima K, Yohda M. “Characterization of Archaeal
Group II Chaperonin-ADP-Metal Fluoride Complexes:
IMPLICATIONS THAT GROUP II CHAPERONINS OPERATE AS A "TWO-STROKE
ENGINE".” J Biol Chem. 280, 40375-83 (2005). |
|
(42) |
Hirose
M., Tohda H., Giga-Hama Y., |
|
(43) |
Ayabe K., Zako T., Ueda H. ”The role of firefly luciferase
C-terminal domain in efficient coupling of adenylation
and oxidative steps.”, FEBS Lett. 579, 4389-94
(2005). |
|
(44) |
Zhang
G-J., Tanii T., Zako T., Hosaka T., Miyake T., Kanari
Y., Funatsu T., |
|
(45) |
Zako T., Iizuka R., Okochi M., Nomura
T., Ueno T., Tadakuma H., Yohda
M. and Funatsu T.: “Facilitated release of
substrate protein from prefoldin by chaperonin” FEBS Letters 579, 3718-3724 (2005). |
|
(46) |
Zako T., Funatsu T. and Yohda M.:
"Kinetic analysis of interactions between archaeal
prefoldin and chaperonin"
Recent Research Development in Biophysics, 3, 475-483 (2004). |
|
(47) |
Okochi M.,
Nomura T., Zako T., Arakawa T., Iizuka
R., Ueda H., Funatsu T., Leroux
M., Yohda M."Kinetics and binding site for
interaction of prefoldin with groupII
chaperonin: contiguous non-native substrate and chaperonin binding sites in archaeal
prefoldin" J Biol
Chem. 279, 31788-31795 (2004). |
|
(48) |
Yoshida,
T., K. Usui, R. Iizuka,
T. Zako, and M. Yohda.
"Reaction mechanism of archaeal molecular
chaperones" Tanpakushitsu Kakusan
Koso 49, 858-61 (2004). |
|
(49) |
Iizuka R., So
S., Inobe T., Yoshida T., Zako
T., Kuwajima K., Yohda M.
"Role of the helical protrusion in the conformational change and
molecular chaperone activity of the archaeal group
II chaperonin." J Biol
Chem. 279, 18834-18839 (2004). |