Research topics
Development of thermally activated delayed fluorescence (TADF)-type metal complexes
Three-coordinate copper (I) complexes possessing an arylthiolate ligand (-SPh = benzenethiolate) were synthesized and characterized for OLEDs. Efficient blue emission with quantum yields of ~1.0 are observed from complexes at both 293 K and 77 K in the solid state. Molecular orbital (MO) calculations revealed that the major transitions (∼95 %) that contribute to emission were attributed to two types of ligand-to-ligand charge-transfer (LLCT) processes. Small S1-T1 gaps (ΔE(S1-T1) ≈ 600 cm-1), obtained by fitting the temperature dependence of lifetimes, strongly indicate that emission in the solid state at room temperature can be ascribed to TADF, which occurs when the S1-T1 gap is small enough to achieve a thermal equilibrium between the two states [1].
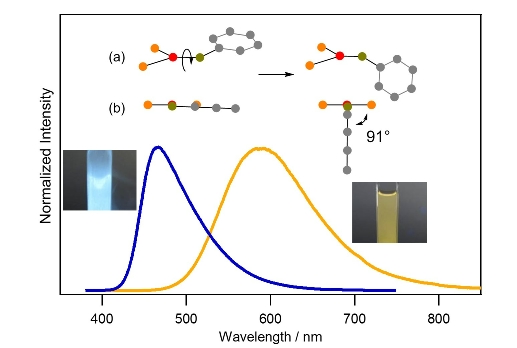
Luminescence color alteration (blue green → orange) and
structural change (the rotation of the aryl ring in Fig. a and b)
Furthermore, we observed that TADF is orange in solutions at room temperature. MO calculatoins indicate that this color alteration is based on the rotation of the -SPh-containing aryl ring about the axis of the Cu-S bond.
- [1] "Highly efficient bule-green delayed fluorescence from copper(I) thiolate complexes: luminescence color alteration by orientation change of the aryl ring", M. Osawa, Chem. Comm. 50, 1801-1803 (2014).
